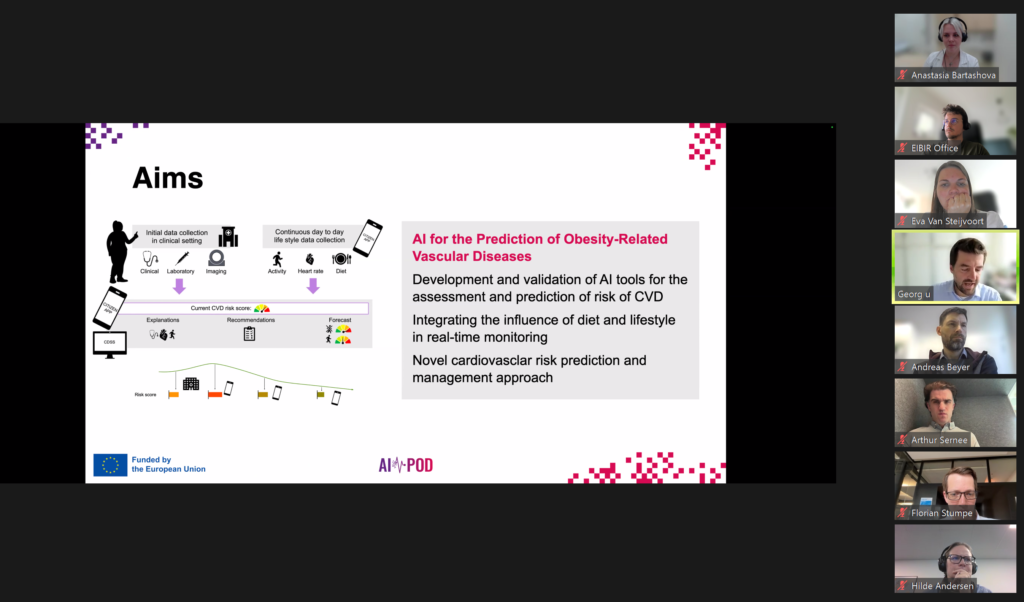
A joint meeting between the Stakeholder Board and External Advisory Board was recently held to review the progress of the AI-based cardiovascular risk assessment project. The session provided an opportunity to present updates across several work packages and gather valuable input on scientific, clinical, and ethical aspects of the work.
Discussions focused on recent developments in the creation of a novel risk scoring system, a clinical decision support tool, and a mobile application designed for citizen engagement. Updates also included advancements in data collection methods, integration of lifestyle information via wearable technologies, and ongoing technical challenges.
Ethical considerations were a recurring theme, particularly regarding data use, potential biases in AI models, and responsible communication of risk to users. Emphasis was also placed on the need to benchmark the project’s tools against established cardiovascular risk models.
Programme agenda:
9:00 – 09:05 – Welcome and Project Progress Overview – A. Bartashova (Medical University of Vienna)
Update on Technical Innovations
09:05 – 09:15 – Risk Score – G. Langs (Medical University of Vienna)
09:15 – 09:25 – CDSS Dashboard – F. Stumpe (medicalvalues GmbH)
09:25 – 09:35 – AI-POD Citizen App – A. Sernee (Brightfish B.V.)
09:35 – 09:45 – AI-POD Clinical Study Update – A. Bartashova (Medical University of Vienna)
09:45 – 09:55 – AI-POD Survey: Stakeholder Perspectives – E. Van Steijvoort (Katholieke Universiteit Leuven)
09:55 – 10:25 – Open Discussion – All participants
10:25 – 10:30 – Closing Remarks – A. Bartashova (Medical University of Vienna)
Key takeaways from the meeting:
Continued progress is being made across technical, clinical, and ethical work streams.
Feedback mechanisms and user-centred improvements remain a priority.
Accessibility and inclusivity are being actively considered in the design of digital tools.
Ethical discussions will continue to be integrated throughout the project’s lifecycle.
Benchmarking efforts are underway to ensure clinical relevance and robustness.
The project team would like to thank both the Stakeholder and External Advisory Boards for their continued engagement, valuable insights, and expert guidance. Their contributions are essential to ensuring the project progresses steadily and remains aligned with clinical needs, user expectations, and ethical best practices.